PROLASTIN-C LIQUID is indicated for adults diagnosed with a severe genetic alpha1-antitrypsin (AAT) deficiency who have clinical evidence of emphysema1
#1 prescribed in ALPHA-1: Trust that comes from experience2
#1 prescribed alpha-1 therapy for more than 25 years2
Experience of more than 4 million infusions worldwide2
>90% success rate in obtaining coverage and the Assist $0 copay program providing up to $6600/year for eligible patients3
One dedicated coordinator specifically assigned to support you and your patients for a seamless transition3
Automatic enrollment in an alpha-1 disease management program proven to impact compliance and healthcare utilization3,4
Treat your patients with alpha-1 with PROLASTIN-C LIQUID
Raising AAT levels is critical for your patients with alpha-1. PROLASTIN-C LIQUID can help.1
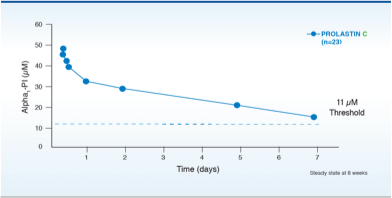
Although the maintenance of blood serum levels of Alpha1-PI (antigenically measured) above 11 µM has been historically postulated to provide therapeutically relevant anti-neutrophil elastase protection, this has not been proven

IMPORTANT SAFETY INFORMATION
PROLASTIN®-C LIQUID is an alpha1-proteinase inhibitor (human) (alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinical evidence of emphysema due to severe hereditary deficiency of alpha1-PI (alpha1-antitrypsin deficiency).
Limitations of Use
- The effect of augmentation therapy with any alpha1-PI, including PROLASTIN-C LIQUID, on pulmonary exacerbations and on the progression of emphysema in alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials
- Clinical data demonstrating the long-term effects of chronic augmentation or maintenance therapy with PROLASTIN-C LIQUID are not available
- PROLASTIN-C LIQUID is not indicated as therapy for lung disease in patients in whom severe alpha1-PI deficiency has not been established
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA)-deficient patients with antibodies against IgA or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-PI products.
Hypersensitivity reactions, including anaphylaxis, may occur. Monitor vital signs and observe the patient carefully throughout the infusion. If hypersensitivity symptoms occur, promptly stop PROLASTIN-C LIQUID infusion and begin appropriate therapy.
Because PROLASTIN-C LIQUID is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
The most common adverse reactions during PROLASTIN-C LIQUID clinical trials in >5% of subjects were diarrhea and fatigue, each of which occurred in 2 subjects (6%).
Please see full Prescribing Information for PROLASTIN-C LIQUID.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
1. PROLASTIN®-C LIQUID (alpha1-proteinase inhibitor [human]) Prescribing Information. Grifols. 2. Data on file, Grifols. 3. Data on file, PROLASTIN DIRECT program, Grifols. 4. Campos MA, Alazemi S, Zhang G, Wanner A, Sandhaus RA. Effects of a disease management program in individuals with alpha-1 antitrypsin deficiency. COPD. 2009;6(1):31-40.


