

Enroll your patients in the PROLASTIN DIRECT® program
With PROLASTIN DIRECT, the exclusive source for PROLASTIN-C LIQUID, your patients receive benefits beyond therapy with ongoing, comprehensive, personalized alpha-1 support.1
Order your free AlphaID™ screening kits
Screen all your patients with COPD (chronic obstructive pulmonary disease) with the new AlphaID, a quick and easy cheek swab for screening your patients for alpha-1. AlphaID is completely free and confidential, from ordering to results.
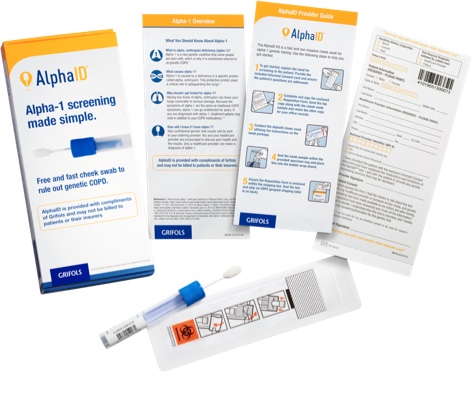
The receipt of this free testing service does not create any expectation or obligation on your part to purchase or use any product or service offered by any manufacturer.
Why PROLASTIN-C LIQUID?
PROLASTIN-C LIQUID provides a convenient, ready-to-infuse liquid formulation that has been proven to effectively raise alpha1-antitrypsin protein levels in patients with alpha-1.2
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA) deficient patients with antibodies against IgA, or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-proteinase (human) products.
About alpha-1
Alpha1-antitrypsin deficiency (alpha-1) is the #1 known genetic risk factor for COPD and may be a contributing cause for up to 3% of COPD cases in the United States.3,4

IMPORTANT SAFETY INFORMATION
PROLASTIN®-C LIQUID is an alpha1-proteinase inhibitor (human) (alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinical evidence of emphysema due to severe hereditary deficiency of alpha1-PI (alpha1-antitrypsin deficiency).
Limitations of Use
- The effect of augmentation therapy with any alpha1-PI, including PROLASTIN-C LIQUID, on pulmonary exacerbations and on the progression of emphysema in alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials
- Clinical data demonstrating the long-term effects of chronic augmentation or maintenance therapy with PROLASTIN-C LIQUID are not available
- PROLASTIN-C LIQUID is not indicated as therapy for lung disease in patients in whom severe alpha1-PI deficiency has not been established
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA)-deficient patients with antibodies against IgA or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-PI products.
Hypersensitivity reactions, including anaphylaxis, may occur. Monitor vital signs and observe the patient carefully throughout the infusion. If hypersensitivity symptoms occur, promptly stop PROLASTIN-C LIQUID infusion and begin appropriate therapy.
Because PROLASTIN-C LIQUID is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
The most common adverse reactions during PROLASTIN-C LIQUID clinical trials in >5% of subjects were diarrhea and fatigue, each of which occurred in 2 subjects (6%).
Please see full Prescribing Information for PROLASTIN-C LIQUID.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
1. Data on file, PROLASTIN DIRECT program. 2. PROLASTIN®-C LIQUID (alpha1-proteinase inhibitor [human]) Prescribing Information. Grifols. 3. World Health Organization. α1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Org. 1997;75(5):397-415. 4. Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with α1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128(3):1179-1186.

