Alpha₁-antitrypsin deficiency, or “alpha-1,” is a rarely diagnosed genetic condition often called genetic COPD.
Alpha-1 is passed from generation to generation, and it can affect your lungs and/or liver. Only genetic testing by a doctor can confirm a diagnosis of alpha-1.
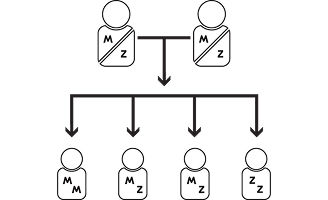
Alpha-1 is passed down through families.
Everyone inherits an alpha-1 gene from each parent. Individuals who inherited two normal M genes do not have alpha-1, while those who inherited two abnormal genes are considered to have alpha-1. Among the many gene variants, the S and Z abnormalities are common. Some individuals inherit one normal M gene and one abnormal gene, such as S or Z. For example, parents with an MZ genetic makeup can pass the abnormal Z gene to their children.
MM
Does not carry an abnormal alpha-1 gene and does not have alpha-1
MZ
Carries one abnormal alpha-1 gene
ZZ
Has two abnormal alpha-1 genes and has alpha-1
People diagnosed with alpha-1 should encourage family members to get tested too.3-5
Alpha-1 is a genetic condition. If a family member is diagnosed with alpha-1, or found to have one abnormal alpha-1 gene, other family members could be affected. Since alpha-1 is a progressive disease, even if they don’t have symptoms now, early diagnosis could help prevent future lung damage.6

Help family members learn their risk of alpha-1 for free.
Only a doctor can confirm a diagnosis of alpha-1, but family members can screen themselves for the risk of alpha-1, at home and at no cost.* Learn more at AlphaIDatHome.com.
*The receipt of this free testing service does not create any expectation or obligation to purchase or use any product or service offered by any manufacturer.
Alpha-1 can feel overwhelming, but little changes can make a big difference.
Developing the right habits could benefit your overall health.
Important Safety Information
PROLASTIN®-C LIQUID is an alpha1-proteinase inhibitor (human) (alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinical evidence of emphysema due to severe hereditary deficiency of alpha1-PI (alpha1-antitrypsin deficiency).
Limitations of Use
- The effect of augmentation therapy with any alpha1-PI, including PROLASTIN-C LIQUID, on pulmonary exacerbations and on the progression of emphysema in alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials
- Clinical data demonstrating the long-term effects of chronic augmentation or maintenance therapy with PROLASTIN-C LIQUID are not available
- PROLASTIN-C LIQUID is not indicated as therapy for lung disease in patients in whom severe alpha1-PI deficiency has not been established
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA)-deficient patients with antibodies against IgA or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-PI products.
Hypersensitivity reactions, including anaphylaxis, may occur. Monitor vital signs and observe the patient carefully throughout the infusion. If hypersensitivity symptoms occur, promptly stop PROLASTIN-C LIQUID infusion and begin appropriate therapy.
Because PROLASTIN-C LIQUID is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
The most common adverse reactions during PROLASTIN-C LIQUID clinical trials in >5% of subjects were diarrhea and fatigue, each of which occurred in 2 subjects (6%).
Please see full Prescribing Information for PROLASTIN-C LIQUID.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit http://www.fda.gov/medwatch, or call 1-800-FDA-1088.
References
- Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with α1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128(3):1179-1186.
- Alpha-1 Foundation. Testing & Diagnosis, https://alpha1.org/testing-diagnosis/ Accessed 12 May 2025.
- World Health Organization. α1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Org. 1997;75(5):397-415.
- American Thoracic Society/European Respiratory Society statement: Standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818-900.
- Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis. 2016;3(3):668-682.
- AlphaNet. What Does it Mean to be an Alpha-1 Advocate? https://www.alphanet.org/what-does-it-mean-to-be-an-alpha-1-advocate. Accessed 12 May 2025.






