Enroll your patients in the PROLASTIN DIRECT® program
With PROLASTIN DIRECT, the exclusive source for PROLASTIN-C LIQUID, you and your patients receive benefits beyond therapy with ongoing, comprehensive, personalized alpha-1 support.1
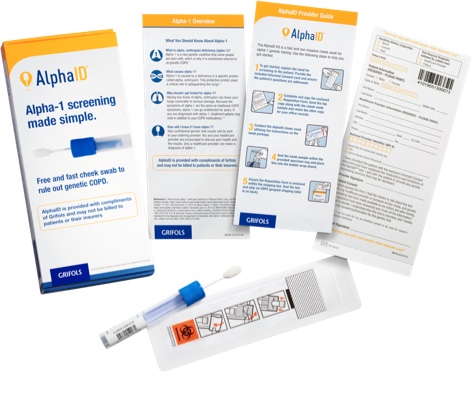
Order your free AlphaID™ screening kits
Screen all your patients with COPD (chronic obstructive pulmonary disease) with the new AlphaID, a quick and easy cheek swab for screening your patients for alpha-1. AlphaID is completely free and confidential, from ordering to results.
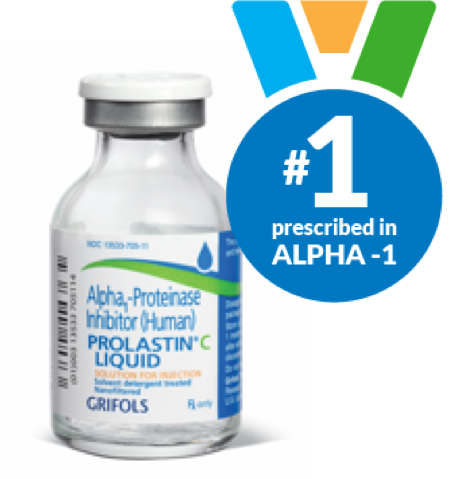
Why PROLASTIN-C LIQUID?
PROLASTIN-C LIQUID provides a convenient, ready-to-infuse liquid formulation that has been proven to effectively raise alpha1-antitrypsin protein levels in patients with alpha-1.2
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA) deficient patients with antibodies against IgA, or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-proteinase (human) products.
About alpha-1
Alpha1-antitrypsin deficiency (alpha-1) is the #1 known genetic risk factor for COPD and may be a contributing cause for up to 3% of COPD cases in the United States.3,4

IMPORTANT SAFETY INFORMATION
PROLASTIN®-C LIQUID is an alpha1-proteinase inhibitor (human) (alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinical evidence of emphysema due to severe hereditary deficiency of alpha1-PI (alpha1-antitrypsin deficiency).
Limitations of Use
- The effect of augmentation therapy with any alpha1-PI, including PROLASTIN-C LIQUID, on pulmonary exacerbations and on the progression of emphysema in alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials
- Clinical data demonstrating the long-term effects of chronic augmentation or maintenance therapy with PROLASTIN-C LIQUID are not available
- PROLASTIN-C LIQUID is not indicated as therapy for lung disease in patients in whom severe alpha1-PI deficiency has not been established
PROLASTIN-C LIQUID is contraindicated in immunoglobulin A (IgA)-deficient patients with antibodies against IgA or patients with a history of anaphylaxis or other severe systemic reaction to alpha1-PI products.
Hypersensitivity reactions, including anaphylaxis, may occur. Monitor vital signs and observe the patient carefully throughout the infusion. If hypersensitivity symptoms occur, promptly stop PROLASTIN-C LIQUID infusion and begin appropriate therapy.
Because PROLASTIN-C LIQUID is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
The most common adverse reactions during PROLASTIN-C LIQUID clinical trials in >5% of subjects were diarrhea and fatigue, each of which occurred in 2 subjects (6%).
Please see full Prescribing Information for PROLASTIN-C LIQUID.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
1. Data on file, PROLASTIN DIRECT program. 2. PROLASTIN®-C LIQUID (alpha1-proteinase inhibitor [human]) Prescribing Information. Grifols. 3. World Health Organization. α1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ. 1997;75(5):397-415. 4. Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with α1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128(3):1179-1186.